Ancient microbial life used arsenic to thrive in a world without oxygen
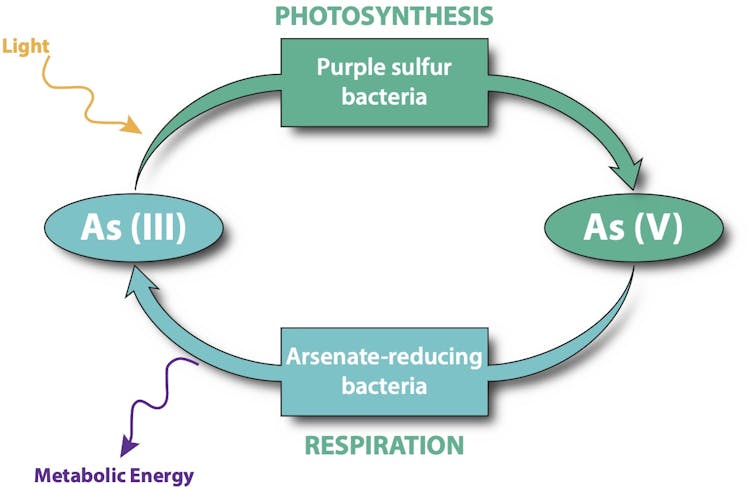
How ancient microbes survived in a world without oxygen has been a mystery. Scientists discovered a living microbial mat that uses arsenic instead of oxygen for photosynthesis and respiration.