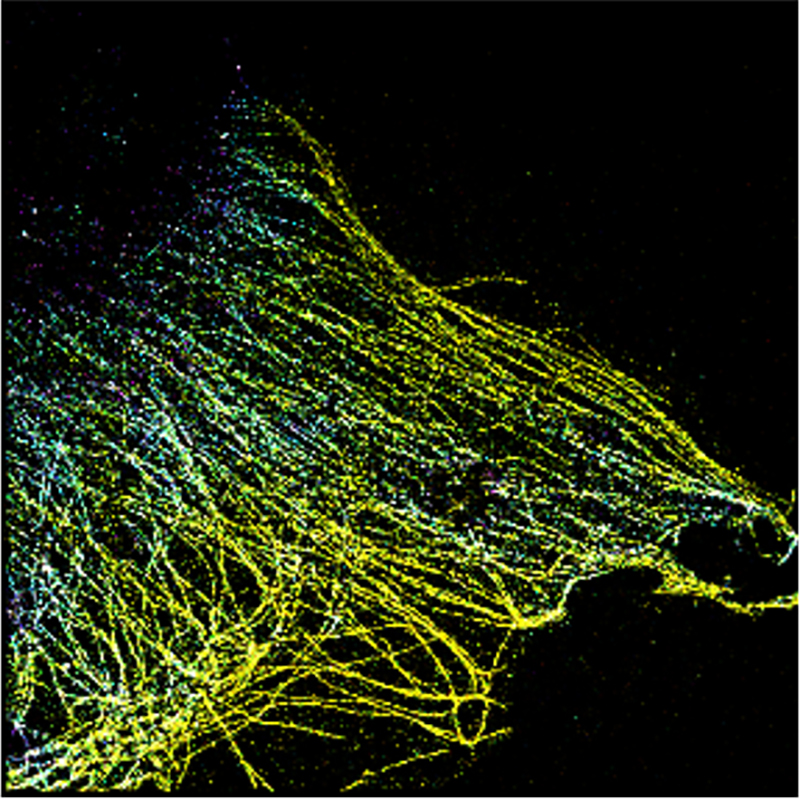
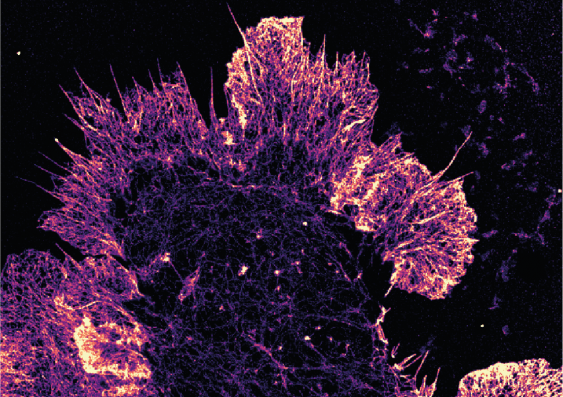
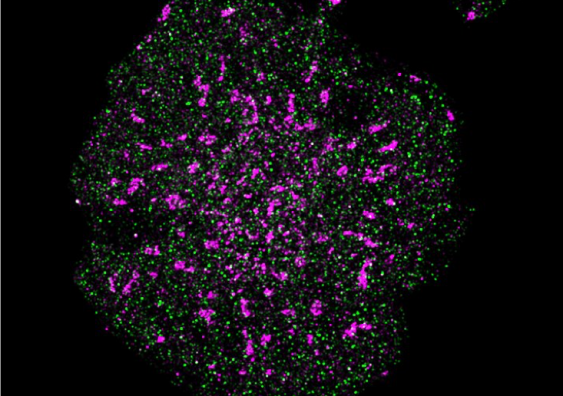
The new technique pioneered by UNSW researchers paves the way for advances in our understanding of complex cellular processes, and could make expensive research a lot cheaper.
If you want to study different parts of a cell at the same time, multicolour fluorescence imaging is really the only game in town.
It has allowed us to examine with great precision cancerous and pre-cancerous tissues in clinical samples, how immune cells respond to infection, and how cell-types interact in systems as complicated as the human body.
Scientists usually add fluorescent dyes (fluorophores) to samples, then fire lasers on them to make the dyes glow, which helps distinguish different parts of what they’re analysing.
Those typical methods tend to fall down, however, when you want to look at multiple parts of a cell at once – those fluorophores have overlapping colours so it can be tricky to tell them apart.
Multiple colours mean multiple lasers, and that’s when things start to get expensive.
UNSW Scientia Professor Justin Gooding and his team from the School of Chemistry have come up with a simple solution which could have massive implications for biological and medical research.
“It allows researchers to do multicolour images who would normally not have the funds to buy the expensive microscopes required for multi-laser microscopes,” Prof. Gooding said.
“It lets us see inside cells like never before.”
The new technique relies on electrochemical fluorescence modulation, and it dramatically increases the number of colours you can see on a standard microscope.
Simply put, scientists use a clear electrode – in this case an indium tin oxide-coated glass coverslip – and adjust the voltage in the experiment.
That's how they discovered the fluorescence changes in predictable ways.
They call this the “electrochemical spectrum” and outline it in paper published recently in Nature Photonics.
So, what’s it to you?
Quite a lot, in fact.
Professor Gooding’s process is a lot simpler – it doesn’t require brand new, ultra high-tech microscopes, opening up a lot of possibilities, including understanding complicated cellular processes and doing things like cutting-edge cancer research and diagnosis.
Professor Maria Kavallaris is Conjoint Professor at UNSW Medicine & Health, and a cancer researcher at Children’s Cancer Institute – she wasn’t involved in the study, but said this work was very exciting.
“It’s got the potential to be used in the diagnosis of diseases,” she said.
“This technology will be valuable when studying cell biology and diseases enabling the localisation of protein expression in tissues using a simple, yet sophisticated technology,” she said.
The device itself is a pretty simple attachment for the types of microscopes most research institutions probably already have.
Prof. Gooding is in discussions with major microscope manufacturers and said it could be available relatively soon.
“With the right partner, I expect this could be available within two years,” he said.
Media enquiries
For media enquiries, please contact Tom Melville, UNSW Science News and Content Coordinator
Tel: +61 432 912 060
Email: tom.melville@unsw.edu.au