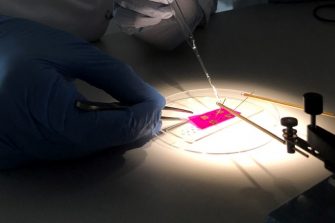
Polymers are a range of materials that are composed of long chains of repeating molecules called monomers.
The long molecular chains intertwine to form complex compositional arrangements.
The backbone of these long molecules is the carbon, C, atom. From valence theory it is known that carbon has 4 electrons in its outer shell. It can therefore share a further 4 electrons to complete its atomic structure. In order to achieve this it must covalently bond with other atoms around it.
One of the more common atoms that carbon is likely to bond with is hydrogen, H.
It is known that hydrogen will share 1 electron to complete its outer shell, therefore carbon will bond with 4 hydrogen atoms
However, polymers are supposed to be made up of long chain molecules. So, how are they made?